Rentox, a pharmaceutical product that has been gaining attention in the market, has raised questions about its regulatory approval status. As consumers and healthcare professionals seek reliable information, it is crucial to understand the process of FDA approval and the current standing of Rentox. This comprehensive article will explore the various aspects of FDA approval, the status of Rentox, and the potential implications for its usage.
Table of Contents
ToggleUnderstanding FDA Approval

What Does FDA Approval Mean?
The Food and Drug Administration (FDA) is the regulatory body responsible for ensuring the safety and efficacy of pharmaceutical products in the United States. FDA approval is a rigorous process that involves extensive testing, clinical trials, and a thorough review of the product’s data by the agency. When a pharmaceutical product receives FDA approval, it means that the FDA has determined the product to be safe and effective for its intended use, based on the available scientific evidence.
Importance of FDA Approval in Pharmaceuticals
FDA approval is crucial in the pharmaceutical industry for several reasons. It provides a level of assurance to consumers and healthcare providers that the product has been thoroughly evaluated and meets the necessary standards for safety and efficacy. This, in turn, fosters trust in the healthcare system and helps to protect public health. Additionally, FDA approval is often a prerequisite for insurance coverage and reimbursement, making it an essential step for pharmaceutical companies to ensure the commercial viability of their products.
The Process of FDA Approval

Clinical Trials and Testing Requirements
The FDA approval process begins with extensive preclinical testing, followed by a series of clinical trials. Pharmaceutical companies must conduct rigorous studies to demonstrate the safety and efficacy of their products, involving various phases of testing with increasingly larger patient populations. These trials are designed to evaluate the drug’s effectiveness, potential side effects, and overall risk-benefit profile.
Review Process by the FDA
Once the clinical trial data is compiled, the pharmaceutical company submits a comprehensive New Drug Application (NDA) to the FDA. The FDA then undertakes a thorough review of the submitted data, examining the product’s safety, efficacy, and manufacturing processes. This review process can take several months, during which the FDA may request additional information or studies from the company.
Post-Market Surveillance
Even after a product receives FDA approval, the regulatory oversight does not end. The FDA continues to monitor the product’s safety and performance through post-market surveillance, which includes monitoring adverse event reports, conducting ongoing studies, and reviewing any new information that comes to light. This ensures that the product remains safe and effective for its intended use.
Current Status of Rentox

Available Information on Rentox’s FDA Status
At the moment, there is limited publicly available information on the FDA approval status of Rentox. The product’s manufacturer has not yet announced whether Rentox has received FDA approval or is currently undergoing the approval process. This lack of clear information has led to uncertainty and speculation among consumers and healthcare professionals.
Expert Opinions on Rentox’s Approval Chances
Given the limited information, it is difficult to make a definitive assessment of Rentox’s chances of obtaining FDA approval. However, some industry experts have cautiously speculated that the product may face challenges in the approval process, as the FDA typically requires robust clinical data to demonstrate the safety and efficacy of new pharmaceutical products. The level of scrutiny and the specific requirements for Rentox’s approval may vary depending on the product’s intended use and the regulatory landscape.
Benefits of FDA Approval

Safety and Efficacy Assurance
The primary benefit of FDA approval is the assurance it provides regarding the safety and efficacy of a pharmaceutical product. The rigorous review process ensures that the product has been thoroughly evaluated and meets the necessary standards for safe and effective use. This gives healthcare providers and consumers confidence in the reliability of the product.
Market Acceptance and Trust
FDA approval also plays a crucial role in the market acceptance and trust of a pharmaceutical product. Consumers and healthcare professionals often perceive FDA-approved products as more trustworthy and reliable, which can significantly impact their willingness to use or prescribe the product. This, in turn, can enhance the commercial success and widespread adoption of the approved product.
Potential Risks of Non-Approval
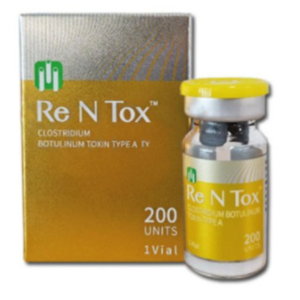
Legal Implications for Usage
In the absence of FDA approval, the legal status of a pharmaceutical product can be uncertain. The use of unapproved drugs may be subject to legal restrictions, and healthcare providers may face liability concerns when prescribing or administering such products. This can create significant challenges for the product’s distribution and usage.
Health Risks Associated with Unapproved Drugs
Unapproved drugs may pose potential health risks to consumers, as their safety and efficacy have not been thoroughly evaluated by the FDA. Without the regulatory oversight and post-market surveillance that comes with FDA approval, there is a higher risk of adverse events, unexpected side effects, or suboptimal therapeutic outcomes.
Alternatives to Rentox
Other FDA Approved Treatments
If Rentox is not approved by the FDA, consumers and healthcare providers may need to explore alternative FDA-approved treatments for the same or similar conditions. These approved products have undergone the necessary testing and evaluation to ensure their safety and effectiveness, providing a more reliable option for patients.
Comparison of Effectiveness and Safety
When considering alternatives to Rentox, it is important to compare the effectiveness and safety profiles of the approved products to determine the most suitable option for the patient’s needs. This comparison should be based on the available clinical data and expert guidance to ensure informed decision-making.
Public Perception of Rentox
Consumer Confidence in FDA Approved Medications
The public’s perception of pharmaceutical products is heavily influenced by the FDA’s approval status. Consumers generally have a higher level of trust and confidence in medications that have received FDA approval, as they perceive these products to be more reliable and safer than unapproved alternatives.
Social Media Impact on Rentox’s Reputation
In the digital age, social media plays a significant role in shaping public opinion. The lack of clear information about Rentox’s FDA approval status may lead to speculation and discussion on various social media platforms. This could potentially impact the product’s reputation and public perception, both positively and negatively, depending on the nature of the online discourse.
Regulatory Challenges for Rentox
Factors Affecting Approval Process
The approval process for pharmaceutical products can be influenced by various factors, such as the nature of the product, the target patient population, the availability of alternative treatments, and the regulatory landscape. These factors may present unique challenges for Rentox and impact the likelihood of its approval by the FDA.
Competition with Other Products in the Market
Rentox may also face competitive pressures from other FDA-approved products targeting the same or similar conditions. The presence of established and trusted alternatives in the market can make it more challenging for Rentox to gain acceptance and secure regulatory approval.
Conclusion
The FDA approval status of Rentox remains a critical question for consumers, healthcare providers, and the broader pharmaceutical industry. The FDA’s rigorous review process and the importance of regulatory approval in the healthcare landscape make it essential to have a clear understanding of Rentox’s current standing and the potential implications of its approval or non-approval.
As more information becomes available, it will be crucial for stakeholders to closely monitor the regulatory developments surrounding Rentox. The benefits of FDA approval, such as safety and efficacy assurance, as well as the potential risks associated with unapproved products, should be carefully considered. Ultimately, the FDA’s decision on Rentox’s approval will have significant implications for its usage, market acceptance, and the overall trust in the pharmaceutical industry.
Contact us via other platforms if you have any questions or requests that need to be answered quickly.
Tiktok: www.tiktok.com/@lunabeautyacademy6
Hotline: 034 254 0228
Email: lunabeautyacademy@gmail.com
Address: No. 29, Alley 140/1/2, Lane 140 Nguyen Xien, Thanh Xuan, Hanoi
Luna wishes you success and hopes you will have the best experiences at the academy. If you need advice or answers about anything, please leave your Contact Information With Us, the Luna team will contact you soon. Thank you for reading this article